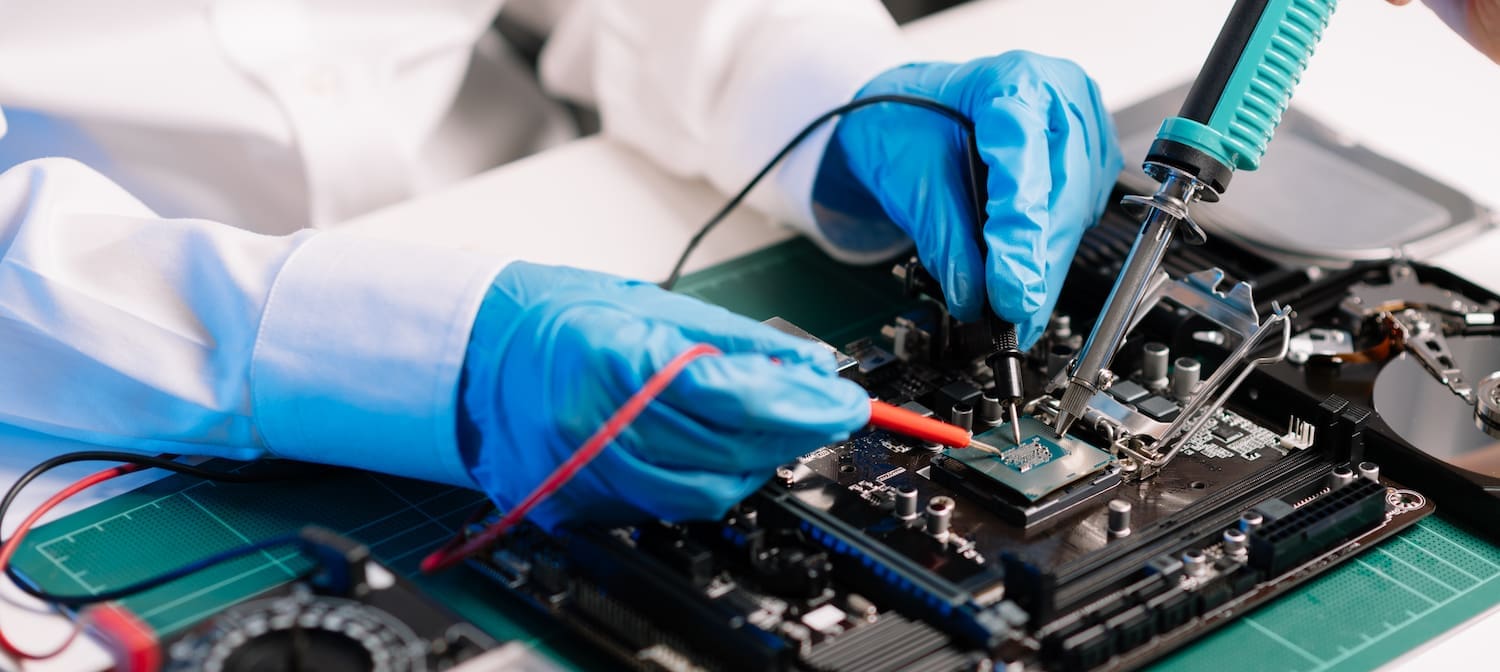
Medical Devices
A medical device is any product or equipment that is intended to diagnose, treat, or prevent a health issue. Their importance is fundamental to improving quality of life. Companies involved in the development, testing, and production of these devices must meet stringent quality standards. Dycem supports leaders in the industry by offering an effective process improvement by installing Dycem mats in cleanrooms for medical devices and other critical areas, dramatically reducing contamination and the damage it can cause.





Where to use Dycem in medical Device Manufacturing
Gown rooms and personnel airlocks.
Packaging areas.
ESD-sensitive areas.
Entry and exits to sterile processing.
Material airlocks.
Product transfer areas.
Contamination: The Risk to Medical Device Manufacturing
Medical devices are divided into different classes with varying levels of quality needs. They range in complexity from a bandage or syringe to an implanted cerebellar stimulator. Depending on the intended use, contamination and unwanted particles within manufacturing can be hugely detrimental. The use of cleanrooms and controlled spaces helps to monitor and minimize the level of contaminants from personnel, equipment, and environmental factors.
Common types of contamination found within manufacturing include dust, fibers, skin cells, bacteria, microorganisms, or other particulate matter. Static (ESD) can also be a contaminant for devices with an electronic component. The presence of a contaminant can cause several negative and costly outcomes including product failure and recalls, and more importantly the risk of infection to patients from a contaminated device.
The risks to a company’s brand, its profitability, and its people are too big to be ignored. Effective contamination control is essential for facilities to ensure the safety of the devices they produce.
Dycem mats, implemented as part of a contamination control strategy, offer optimal support in creating a clean room for medical devices, reducing the risk of contamination in medical device manufacturing.
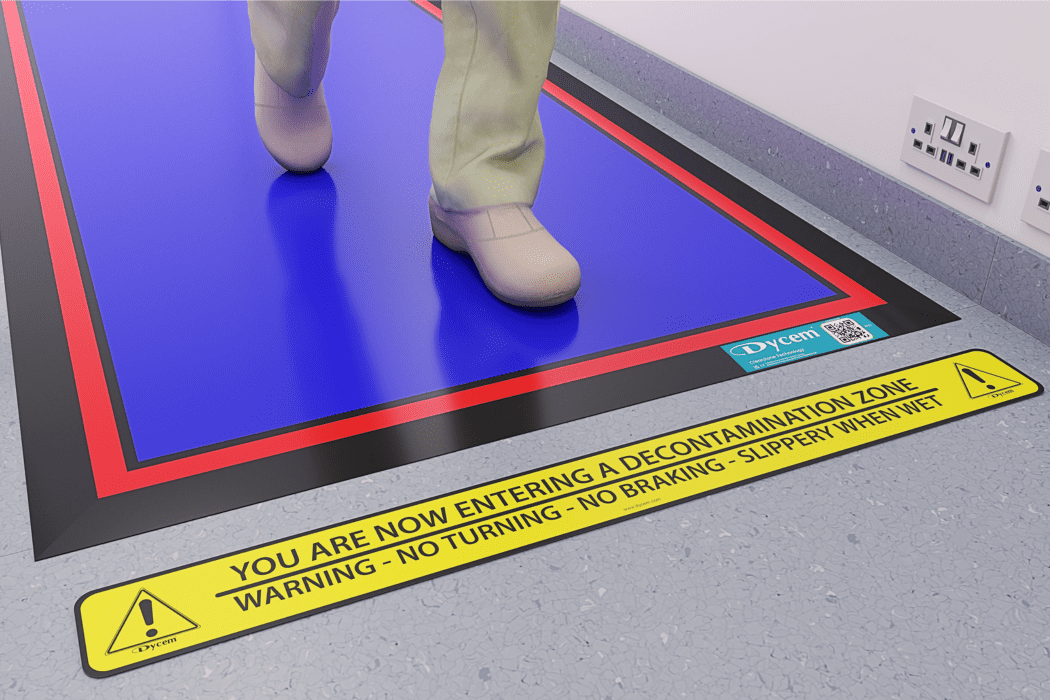
Don't Ignore the Floor...
80% of Contamination Enters a Critical Space at Floor Level.
Benefits of Dycem in Medical Device Manufacturing
Superior Particle Collection & Retention
Particulate from shoes and wheels are captured on Dycem mats and remain on the surface until cleaned off. With Dycem, medical device manufacturers benefit from a reduction in floor-level particulate of up to 99.9% and a reduction in air particulate levels of up to 75%.
REDUCED MICROBIAL CONTAMINATION CONCERNS
With Dycem mats installed prior to critical areas, our medical device customers have peace of mind that damage from microbial contamination is minimized. All Dycem products contain Biomaster, an active antimicrobial additive.

Long-Lasting, Cost-Effective Alternative
Unlike disposable contamination control solutions, Dycem mats last on average 3+ years. Manufacturers typically see an ROI on their cleanroom for medical devices shortly after purchase, as well as the added benefit of a reduced carbon footprint.
Our Products, Your Industry
Medical device manufacturers must take every step necessary to ensure a safe and sterile process to protect their products and ultimately the patients they will help. Dycem offers solutions to accommodate a wide range of devices and address the diverse quality control requirements of the industry. Keeping contamination out of critical spaces and cleanrooms for medical devices is our priority.

Dycem CleanZone
CleanZone helps control contamination from the shoes of personnel.
Dycem Floating Mats
Floating Mats are a contamination control solution that can be moved as needed.
In Your Industry
Discover your industry’s specific applications—all conveniently summarized right here.
Success Stories...
Discover our success stories in your industry.
Learn how our customers have benefitted from installing Dycem.
































WHO's List of Health Threatening Fungi
Check out the World Health Organization’s first-ever list of health-threatening fungi:
“The WHO fungal priority pathogens list (FPPL) is the first global effort to systematically prioritize fungal pathogens, considering the unmet research and development (R&D) needs and the perceived public health importance. The WHO FPPL aims to focus and drive further research and policy interventions to strengthen the global response to fungal infections and antifungal resistance.”